ISPE GAMP® event in Poland
I had the privilege last week to present on the above topic, in an ISPE GAMP® event in Poland. The topic is very relevant as part of an organisation’s holistic control strategy, maintaining the quality and integrity of the records and data throughout the process and lifecycle, linked to your product production.
The utilisation of Electronic Production Records (EPRs) within a Life Science organisation can be used to drive efficiencies, cost savings, improve security across the organisation, while continuing to deliver against regulatory (GxP) requirements and protecting patient safety and product quality.
It is important to understand whether your production records are giving you the best quality information and most efficient means to manage your manufacturing-related data and operations? It’s not just about meeting regulatory records requirements but also supporting your business and personnel activities in efficient ways.
These records have gained more significance to organisations, with digitalization and the use of AI (artificial intelligence) and Digital Maturity to enhance performance in a seamless factory of the future.
At the conference, I asked the audience, about the use of paper versus electronic at their companies. There was still a low response of companies implementing and digitalizing their process operations and records, they admitted to still using paper batch records, that increases the risk of losing the paper batch records and introduces a potential paper nightmare, by relying on external archive organisations such as Iron Mountain for example.
It is my opinion that companies need to embrace innovation and the paradigm shift and become more involved with initiatives from organisations such as the ISPE (International Society for Pharmaceutical Engineering) and in particular Pharma 4.0 and GAMP® COP (Community of Practice).
As explained in the new Appendix S2 ISPE GAMP® 5 Second Edition, we know that electronic records facilitate data accuracy and portability across job functions, departments and even facilities spread around the globe. However, proper planning, design and implementation of electronic systems is essential to achieving efficient and accurate creation and movement of information among disciplines that manage assets and materials, control production, analyze and/or approve results, as well as store and retrieve information (all in a timely fashion). Meeting cGMP standards must be the uncompromised priority, but efficient operations make a business sustainable while meeting the highest standards.

Technology strategies to enable EPR
- Integration of enterprise systems for data exchange
- Interconnectivity and communications among systems and equipment
- Integrated data management (access, storage and retrieval) to support:
- Process Information Management / Data Historian Systems, Multivariate analytical applications, advanced process control, off-line process simulation & optimization
- Use of distributed ledger systems e.g., blockchain and cryptography
The design and implementation of any computerized system, requires a deep understanding of the various types of data being created, modified, or utilized. The diagram provides examples of data types and system actions.
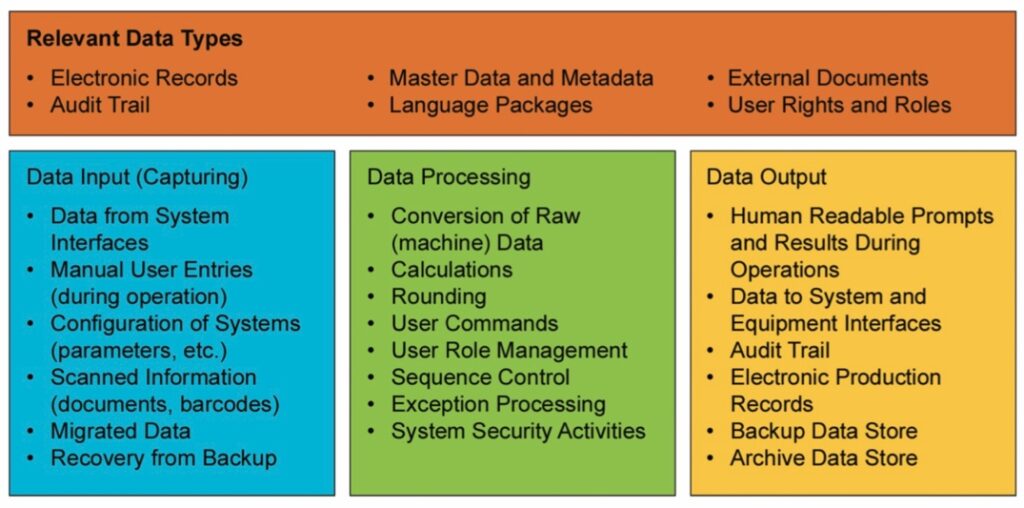
Source ISPE GAMP® 5 Second Edition Appendix S2
For further information on refer to Good Practice Guide GAMP® 5 Second Edition Appendix S2 and GAMP® Good Practice Guide Manufacturing Execution Systems – A Strategic and Program Management Approach Appendices 7 and 8.

